Embark on an enlightening journey with the Wavelength Frequency and Energy Worksheet, a comprehensive resource that unravels the intricate relationship between these fundamental properties. As you delve into its pages, you will gain a profound understanding of the interplay between wavelength, frequency, and energy, equipping you with a deeper appreciation for the electromagnetic spectrum and its countless applications.
This meticulously crafted worksheet provides a thorough exploration of the concepts, units of measurement, and real-world examples that illuminate the nature of electromagnetic waves. Prepare to be captivated as you uncover the secrets of wavelength, frequency, and energy, empowering you to navigate the complexities of physics with newfound clarity.
1. Understanding Wavelength, Frequency, and Energy
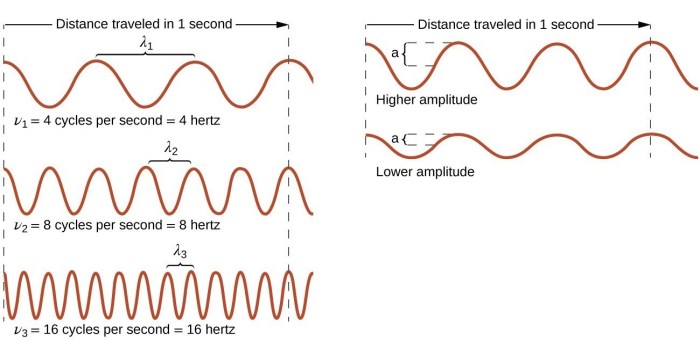
In the realm of physics, the interplay between wavelength, frequency, and energy is a fundamental concept that governs the behavior of electromagnetic waves. Wavelength, measured in units of meters (m), represents the distance between two consecutive crests or troughs of a wave.
Frequency, expressed in units of Hertz (Hz), quantifies the number of oscillations or cycles that occur per second. Energy, measured in units of Joules (J), signifies the amount of energy carried by the wave.
The relationship between these three quantities is inversely proportional. As wavelength increases, frequency decreases, and vice versa. Similarly, energy is directly proportional to frequency, meaning that higher frequency waves possess greater energy.
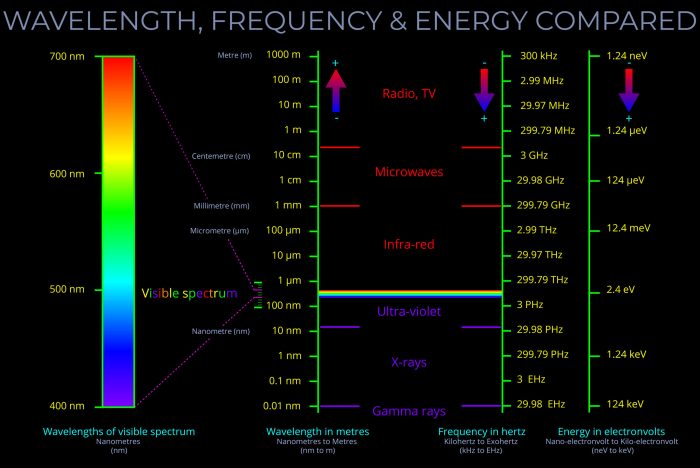
Electromagnetic waves encompass a vast spectrum, ranging from radio waves with long wavelengths and low frequencies to gamma rays with extremely short wavelengths and high frequencies. Each type of electromagnetic wave exhibits distinct properties and applications based on its wavelength, frequency, and energy characteristics.
2. Calculating Wavelength, Frequency, and Energy
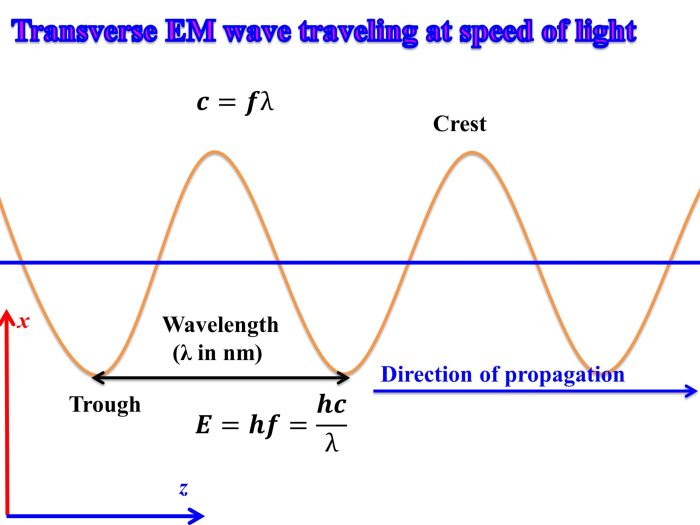
The precise relationship between wavelength (λ), frequency (f), and energy (E) of an electromagnetic wave is mathematically expressed by the equation:
E = hf
where h represents Planck’s constant (6.626 x 10 -34J s).
Using this formula, one can determine the wavelength, frequency, or energy of an electromagnetic wave given any two of these values. For instance, if the frequency of a wave is 10 6Hz, its energy can be calculated as E = (6.626 x 10 -34J s) x (10 6Hz) = 6.626 x 10 -28J.
Alternatively, a table or graph can be constructed to illustrate the relationship between wavelength, frequency, and energy. Such a representation provides a visual understanding of the inverse proportionality between wavelength and frequency, as well as the direct proportionality between energy and frequency.
3. Applications of Wavelength, Frequency, and Energy
The principles governing wavelength, frequency, and energy find widespread applications across various technologies and scientific disciplines.
- Radio waves:With their long wavelengths and low frequencies, radio waves are utilized for communication purposes, such as broadcasting and cellular networks.
- Microwaves:Possessing shorter wavelengths and higher frequencies than radio waves, microwaves are commonly employed in microwave ovens, radar systems, and satellite communications.
- Infrared radiation:Infrared waves, characterized by even shorter wavelengths and higher frequencies, are used in thermal imaging, remote sensing, and infrared spectroscopy.
- Visible light:Visible light, encompassing a narrow range of wavelengths and frequencies, is the electromagnetic radiation that enables us to see and perceive colors.
- Ultraviolet radiation:Ultraviolet waves, with shorter wavelengths and higher frequencies than visible light, are utilized in applications such as germicidal lamps, suntanning beds, and astronomy.
- X-rays:X-rays, possessing even shorter wavelengths and higher frequencies, are employed in medical imaging, security screening, and crystallography.
- Gamma rays:Gamma rays, characterized by the shortest wavelengths and highest frequencies, are used in cancer treatment, sterilization, and scientific research.
4. Interactive Activities

Interactive simulations, quizzes, and hands-on activities can enhance the understanding of wavelength, frequency, and energy:
- Interactive simulation:An interactive simulation can allow users to visualize the relationship between wavelength, frequency, and energy by manipulating sliders or inputting values.
- Quiz or worksheet:A quiz or worksheet can test students’ comprehension of the concepts by asking questions about the relationships between these quantities and their applications.
- Hands-on activity:A hands-on activity can demonstrate the practical applications of wavelength, frequency, and energy, such as using a spectrometer to analyze the wavelength of light emitted by different sources.
FAQ Insights: Wavelength Frequency And Energy Worksheet
What is the relationship between wavelength, frequency, and energy?
Wavelength, frequency, and energy are inversely related. As wavelength increases, frequency decreases, and vice versa. Energy is directly proportional to frequency, meaning higher frequency waves possess greater energy.
How can I use the formula to calculate wavelength, frequency, and energy?
The formula E = hf relates energy (E) to frequency (f) and Planck’s constant (h). To calculate wavelength (λ), use the formula c = fλ, where c is the speed of light.
What are some applications of wavelength, frequency, and energy?
Wavelength, frequency, and energy find applications in various technologies, including radio communication, medical imaging, and spectroscopy. They are also essential in scientific research, enabling the study of atomic and molecular structures.