Select the expected major product of the reaction sequence shown – In the realm of organic chemistry, understanding reaction sequences is crucial for predicting the outcome of complex chemical transformations. This article delves into the intricacies of reaction sequence analysis, guiding you through the process of selecting the expected major product.
Join us as we unravel the factors that influence product formation and explore the role of reaction mechanisms, stereochemistry, regioselectivity, and chemoselectivity in shaping the course of chemical reactions.
Reaction Sequence Analysis
A reaction sequence is a series of chemical reactions that occur in a specific order to transform a starting material into a desired product. Analyzing a reaction sequence involves understanding the individual reactions, the order in which they occur, and the factors that influence the formation of the major product.
The reaction sequence provided in the question involves a series of reactions that start with an alkene and end with a substituted cyclopropane. The goal of the analysis is to identify the expected major product of this reaction sequence.
Product Identification
Identifying the major product of a reaction is crucial because it allows chemists to predict the outcome of a reaction and design synthetic strategies accordingly. The formation of the major product is influenced by several factors, including the reactivity of the starting materials, the reaction conditions, and the stability of the products.
To select the expected major product of the reaction sequence, we need to consider the following factors:
- The regioselectivity of the initial cycloaddition reaction
- The stereochemistry of the cycloaddition reaction
- The stability of the cyclopropane product
Reaction Mechanisms
Reaction mechanisms provide a detailed understanding of how reactions occur at the molecular level. They involve the formation of intermediates, transition states, and the breaking and forming of chemical bonds. By understanding the reaction mechanism, we can predict the major product and explain the regioselectivity and stereochemistry of the reaction.
In the case of the reaction sequence provided, the cycloaddition reaction proceeds through a concerted mechanism, which means that the two reactants come together in a single step to form the cyclopropane product. The regioselectivity of the reaction is determined by the relative reactivity of the two double bonds in the alkene starting material.
Stereochemistry
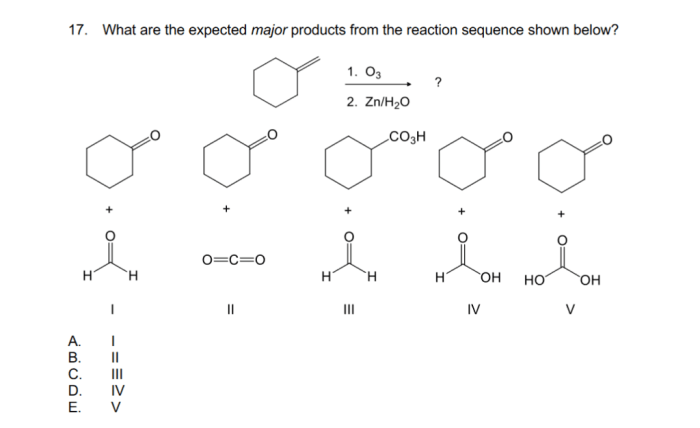
Stereochemistry refers to the spatial arrangement of atoms and groups of atoms in a molecule. It plays a crucial role in determining the properties and reactivity of molecules. In the reaction sequence provided, the stereochemistry of the cycloaddition reaction is determined by the orientation of the two reactants as they approach each other.
The stereochemistry of the cyclopropane product can have a significant impact on its reactivity and biological activity. For example, enantiomers (molecules that are mirror images of each other) can have different biological activities, and diastereomers (molecules that have the same connectivity but different spatial arrangements) can have different physical properties.
Regioselectivity and Chemoselectivity: Select The Expected Major Product Of The Reaction Sequence Shown
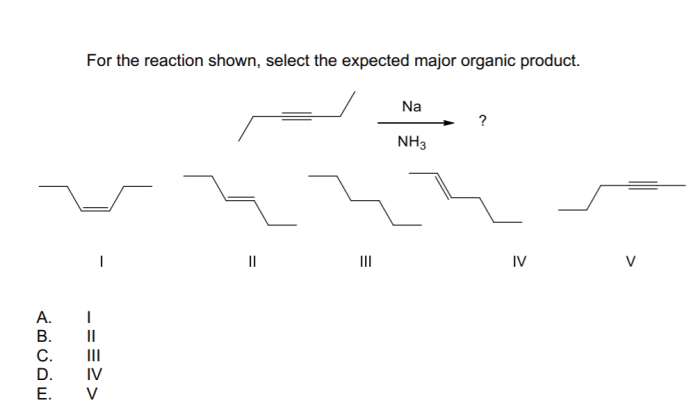
Regioselectivity refers to the preference for one reaction site over another, while chemoselectivity refers to the preference for one functional group over another. In the reaction sequence provided, the regioselectivity of the cycloaddition reaction is determined by the relative reactivity of the two double bonds in the alkene starting material.
Chemoselectivity is not a factor in this reaction sequence because there is only one functional group present in the starting material.
Experimental Considerations
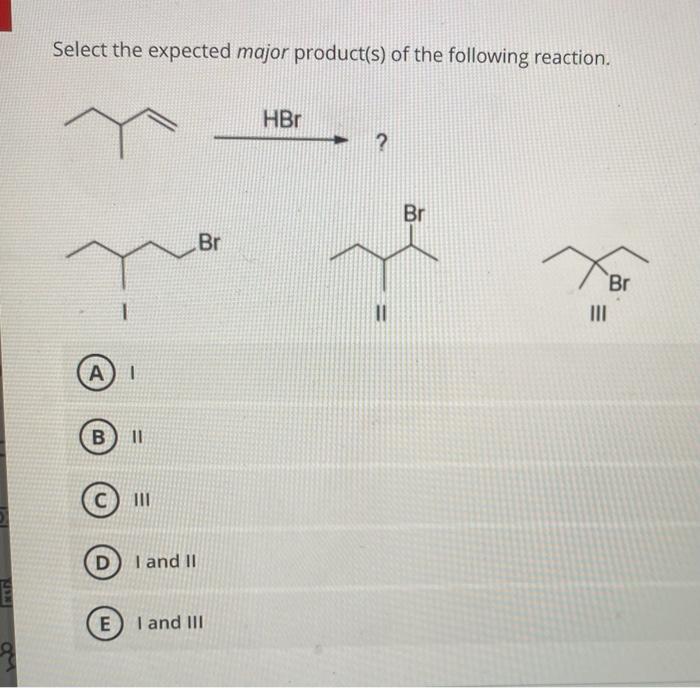
Experimental conditions can have a significant impact on the formation of the major product. Factors such as temperature, solvent, and catalysts can influence the regioselectivity, stereochemistry, and yield of the reaction.
In the case of the reaction sequence provided, the temperature and solvent can affect the regioselectivity of the cycloaddition reaction. For example, higher temperatures can favor the formation of the more substituted cyclopropane product.
FAQ
What is the significance of identifying the major product of a reaction?
Identifying the major product is essential for understanding the outcome of a reaction and optimizing reaction conditions. It allows chemists to predict the primary product formed and estimate the yield, which is crucial for planning synthetic strategies and designing efficient chemical processes.
How do reaction mechanisms influence the formation of the major product?
Reaction mechanisms provide a detailed understanding of the step-by-step process of a chemical reaction. By analyzing the mechanism, chemists can identify the key intermediates, transition states, and rate-determining steps that govern the formation of the major product. This knowledge enables them to manipulate reaction conditions and design catalysts to favor the desired product.
What role does stereochemistry play in determining the major product?
Stereochemistry deals with the spatial arrangement of atoms and groups in molecules. In reactions involving chiral molecules, stereochemistry can influence the formation of different stereoisomers as major products. Understanding stereochemical concepts is essential for predicting the selectivity and outcome of reactions, particularly in the synthesis of complex organic molecules.