Match each description with the appropriate step in enzyme catalysis. This process involves several key steps, including enzyme-substrate complex formation, transition state stabilization, product formation, and enzyme regeneration. Understanding these steps is crucial for comprehending the mechanisms of enzyme-catalyzed reactions.
Enzymes play a vital role in biological systems, facilitating biochemical reactions that are essential for life. By lowering the activation energy required for reactions to occur, enzymes enhance the efficiency and specificity of these processes.
Enzyme Catalysis: Match Each Description With The Appropriate Step In Enzyme Catalysis
Enzyme catalysis is a fundamental process in biochemistry that enables the efficient and specific conversion of substrates into products. Enzymes, which are protein molecules, facilitate chemical reactions by lowering the activation energy required for the reaction to occur. This process involves several key steps, including enzyme-substrate complex formation, transition state stabilization, product formation, and enzyme regeneration.
Enzyme-Substrate Complex Formation
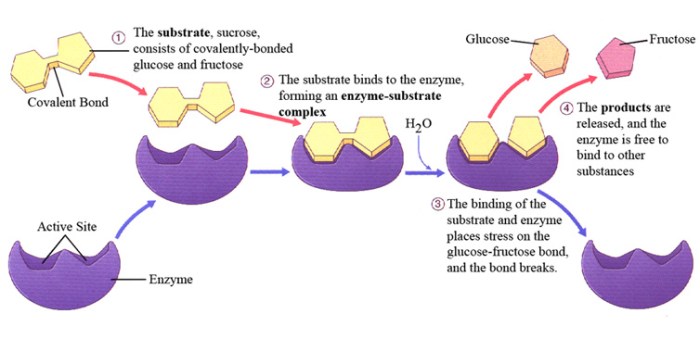
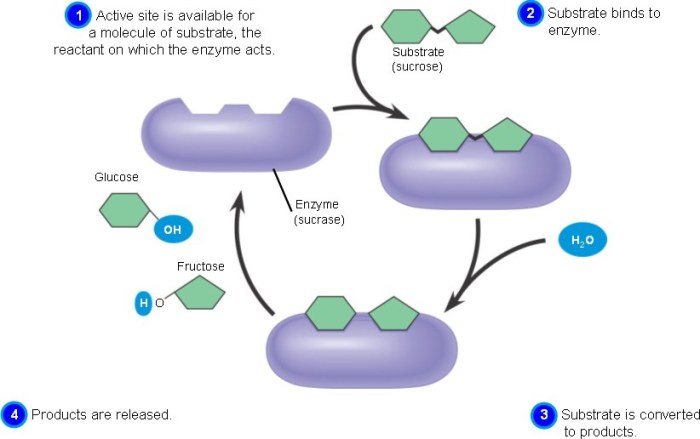
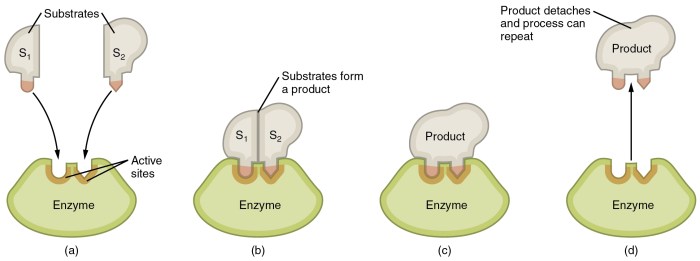
The first step in enzyme catalysis is the formation of an enzyme-substrate complex. The enzyme has a specific binding site, which is a region of the protein that is complementary to the substrate. When the substrate binds to the active site, it forms a non-covalent complex with the enzyme.
This complex is stabilized by various forces, including hydrogen bonds, ionic bonds, and van der Waals interactions.
The binding of the substrate to the active site can induce a conformational change in the enzyme, known as induced fit. This change brings the catalytic groups of the enzyme into close proximity with the substrate, facilitating the catalytic reaction.
Transition State Stabilization
Once the enzyme-substrate complex is formed, the enzyme stabilizes the transition state of the reaction. The transition state is a high-energy intermediate that forms during the conversion of the substrate to the product. Enzymes stabilize the transition state by providing a favorable environment for its formation.
This can be achieved through electrostatic interactions, hydrogen bonding, or other non-covalent interactions.
By stabilizing the transition state, the enzyme lowers the activation energy required for the reaction to occur, making the reaction more efficient.
Product Formation
After the transition state is stabilized, the reaction proceeds to form the product. The catalytic groups of the enzyme facilitate the chemical transformation of the substrate into the product. This process may involve the transfer of electrons, protons, or functional groups.
Once the product is formed, it is released from the enzyme’s active site. This allows the enzyme to bind to another substrate molecule and repeat the catalytic cycle.
Enzyme Regeneration, Match each description with the appropriate step in enzyme catalysis
After the product is released, the enzyme is regenerated to its original state. This process ensures that the enzyme can participate in multiple catalytic cycles. Enzyme regeneration can occur through various mechanisms, including proton transfer, conformational changes, or the binding of cofactors or coenzymes.
The recycling of enzymes is essential for efficient catalysis, as it allows the enzyme to be used multiple times without being consumed in the reaction.
General Inquiries
What is the role of the active site in enzyme catalysis?
The active site is a specific region on the enzyme’s surface that binds to the substrate and facilitates the catalytic reaction. It provides a favorable environment for the substrate to undergo the necessary chemical transformations.
How do enzymes stabilize the transition state?
Enzymes stabilize the transition state by providing a specific environment that lowers the energy barrier for the reaction. This is achieved through interactions between the enzyme and the transition state, such as hydrogen bonding, electrostatic interactions, and van der Waals forces.